Sodium sulfite is a white powder and one of the varieties of sodium mineral salts. Due to its antimicrobial and cleansing properties, it is used in various industries such as paper production, food, and various types of bleaches. Sodium sulfite is an inorganic compound with the chemical formula Na2SO3, which is produced in an anhydrous form and hydrated form (7 water molecules). It is obtained by combining sodium carbonate and sulfur dioxide, and the anhydrous form of this salt melts at 500 degrees Celsius. Sodium without sulfite is transformed into sodium sulfite by reacting with sulfur. This substance is used as a preservative in the production of food and industrial products.
Raag Trading website, with a brilliant track record in commerce, sells and exports sodium sulfite with the highest purity percentage and quality. Therefore, contact our experts and consultants to find out how to buy sodium sulfite and the bulk price of sodium sulfite.
In general, it is not possible to announce a completely fixed price for the bulk purchase of sodium sulfite, as various factors affect the price. These factors include acid concentration, exchange rate fluctuations, oil prices, manufacturer, acid quality, packaging type, as well as fluctuations in the global market for buying and selling sodium sulfite. To find out the current price of sodium sulfite, it is recommended to contact Raag Trading experts. Many factors such as geographical location, exchange rate fluctuations, market conditions, supply and demand level, and supplier company affect the price. Additionally, the purity of this substance also affects the final price. Therefore, it is recommended to consult with our experts before purchasing and exporting sodium sulfite in bulk.
Currently, Raag Ayin Atlas Gharb Company is one of the suppliers of chemical products in the country, providing a wide range of industrial and chemical raw materials from reputable brands to domestic and foreign manufacturing companies. Contact our experts for more information.
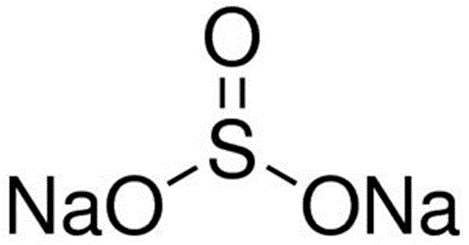
In the figure below, you can see the molecular structure of sodium sulfite.
Sodium sulfite (Na2SO3) is one of the types of salt bases that is obtained from the neutralization reaction between a strong base NaOH and SO2 in the role of a weak acid (hypothetical sulfuric acid) and its aqueous solution is basic with a pH greater than 7.
Sodium sulfite is a white or powdery crystalline substance with a salty taste. It is soluble in water and also dissolves in alcohol in small amounts. Sodium sulfite exists in two forms, dry and crystalline, which decompose upon heating. This substance is easily oxidized and can be used as a reducing agent. Anhydrous sodium sulfite is widely used in industry, but its use is prohibited in foods such as meat and sources of vitamin B1. Since sodium sulfite heptahydrate oxidizes more in the air than anhydrous sodium sulfite, it is also less commonly used. Therefore, when exporting sodium sulfite, it is important to know which type of this substance the customer has ordered.
In the table below, you can see the chemical and physical properties of this substance.
Sodium sulfite is a solid product that becomes active after dissolving in water. Sodium sulfite quickly removes dissolved oxygen in steam boilers, which is a significant advantage in the short term. After removing dissolved oxygen, this product can be converted to sodium sulfate.
The consumption volume of catalytic sodium sulfite in the boiler should be such that its sulfite level is about 20 to 50 ppm, and it should be noted that 10 parts of catalytic sulfite can remove one part of oxygen. Its consumption should be calculated by continuous injection of sodium sulfite through an injection pump. Then, by measuring the amount of sulfite in the steam boiler water, the injection rate can be controlled.
After reviewing what sodium sulfite is, we will examine the types and chemical properties of this substance. The types of sodium sulfite include:
– Food-grade sodium sulfite: Food-grade sodium sulfite is used in the food industry as a preservative for food.
– Industrial-grade sodium sulfite: Industrial-grade sodium sulfite has a wide range of applications in industries such as papermaking, water and wastewater treatment, detergents, and so on.
Please be sure to specify the type of sodium sulfite when placing an order for export.
In the industrial method, sodium sulfite is obtained from the reaction of sulfur dioxide with sodium carbonate solution. Initially, sodium sulfite-free is produced, which is converted to sodium sulfite by reacting with sodium hydroxide or sodium carbonate. Sodium sulfite is decomposed by weak acids, releasing sulfur dioxide gas.
SO2 + Na2CO3 —> Na2SO3 + CO2
In the laboratory method, sulfur dioxide and sodium hydroxide solution are used. During this reaction in warm water, sodium sulfite precipitate is formed, which is dissolved by adding excess sulfur dioxide, and is then converted to disulfite, which crystallizes after cooling. Sodium sulfite is also obtained from the effluent of wood and paper factories.
2NAOH + SO2 —> Na2SO3 + H2O
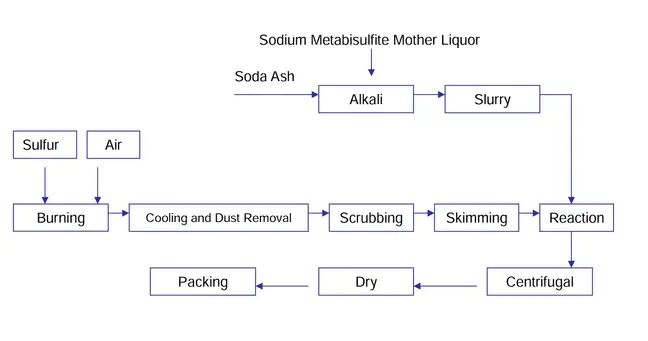
It is worth noting that sodium sulfite is initially prepared and synthesized as a liquid. Then, by using special technology and crystallization equipment, the solution is concentrated, and sodium sulfite crystals are separated and extracted. Finally, these crystals are sent to the drying machine and then packaged. The packaging type for exporting sodium sulfite is explained below.
Two-Step Method
In this method, SO2 gas is passed through a saturated sodium carbonate solution. Initially, sodium sulfite-free solution is prepared. Then, in the second step, sodium sulfite is obtained through neutralization using sodium hydroxide or sodium carbonate solution. Sodium sulfite is extracted from the neutralized solution through crystallization. If the crystallization process is carried out below 35 degrees Celsius, the final product will be Na2SO3,7H2O, which becomes anhydrous sodium sulfite upon heating above 35 degrees Celsius. It is worth noting that the iron level in the sodium carbonate solution should not be less than 3 PPM.
One-Step Method
In this method, a saturated solution of sodium sulfite is first prepared. Then, dry SO2 gas and sodium carbonate are simultaneously added to the solution in stoichiometric amounts, and the reaction occurs. It is essential to charge the carbonate and sulfur dioxide gas accurately and simultaneously, ensuring that the pH of the solution remains in the range of 6.5 to 7.6. Furthermore, the temperature of the solution should be within the range of 35 degrees Celsius, and the maximum level of iron ions should be within 3 PPM.
If the temperature of the sodium sulfite solution increases, it becomes saturated up to the point where iron precipitates and is not visible in the mother liquor. As mentioned earlier, adjusting the pH is crucial. Reducing the pH below 6.5 increases the reaction rate, and there is not enough time for the formation of coarse sodium sulfite crystals. Additionally, a temperature above 35 degrees Celsius and reaching the boiling point in the sodium sulfite solution saturation results in obtaining anhydrous sodium sulfite.
Sodium sulfite exists in various forms, and it is essential to note which type of sodium sulfite the buyer is interested in for export. Each type of this substance exhibits specific reactions, some of which are mentioned below:
Sodium sulfite is easily oxidized and, therefore, is used as a mild reducing agent in various industries.
Sodium sulfite heptahydrate: This type blossoms into crystal form in hot and dry weather. These crystals oxidize in the air and convert into sodium sulfate.
Anhydrous sodium sulfite: This type has higher resistance to oxidation.
Applications of sodium sulfite include many industries, some of which are:
Electrochemical oxidation of metallic silver: One of the issues in the chemical processes of extracting noble metals (especially silver) from waste electronic materials, jewelry and other sources. A complexing agent in the silver oxidation process is sodium sulfite, which solves disadvantages such as the high consumption of reagents and lack of selectivity in its solubility.


Many foreign companies are looking to buy this widely used material. Therefore, to export sodium sulfite or buy it in bulk, you can contact Raag Trading Company
Sodium sulfite is classified as a non-toxic and non-hazardous chemical substance. However, safety precautions should be taken when working with this chemical. Exposure to sodium sulfite can cause skin irritation, eye irritation, and respiratory problems. Inhalation of the substance can cause sensitivity, sore throat, coughing, pulmonary problems, asthma, headaches, and heart irregularities. Prolonged exposure to it can be fatal. When working with chemical substances, a mask with a filter that can remove organic and mineral gases should be used.
If someone inhales toxic chemicals, they should go to an open space and use an oxygen mask if necessary. When working with sodium sulfite catalysts, safety precautions should also be taken. When heated, sodium sulfite produces highly toxic sodium oxide and sulfur oxide vapors. Suitable fire extinguishers, such as water, dry powder, foam, or carbon dioxide, should also be used in case of fire.
If you intend to export sodium sulfite, make sure to follow its safety guidelines and send it in standard and hygienic packaging. RAAG Company offers the safest chemical substances in standard packaging and is a reliable source for buying bulk sodium sulfite and exporting it to other countries.
Sodium sulfite should be stored away from acids and chemical substances in a cool, dry, well-ventilated environment, away from sources of moisture, heat, or sparks. As mentioned, this solid compound is highly sensitive to moisture, so the lid of the container should be tightly closed after each use. It is also necessary to keep these materials away from oxidizing and flammable compounds. The packaging of this product is mainly done in double-layered 25 kg bags (with internal moisture-resistant coating). RAAG Company is one of the reputable centers in the country for the export of sodium sulfite, buying and selling various chemical raw materials and exporting sodium sulfite.
RAAG Company, with years of experience in exporting various food, chemical, and mineral materials, is one of the most reputable companies for buying high-quality and pure sodium sulfite. If you intend to export sodium sulfite or want to send high-quality sodium sulfite to domestic and foreign markets, please contact Raag experts for advice or to buy bulk sodium sulfite and other cleaning materials. Raag, with years of experience in exporting sodium sulfite to various countries, can handle all stages of exporting cleaning raw materials and other chemical substances from scratch to finish.